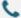As artificial intelligence continues to evolve at an unprecedented pace, AI-driven protein design is emerging as a powerful force in both scientific research and industrial applications. Gone are the days of relying solely on traditional methods to optimize protein functions. AI now enables scientists to design proteins with greater precision and efficiency, revolutionizing fields from biomedicine to environmental sustainability.
In the realm of biomedicine, AI-designed proteins are paving the way for faster vaccine and antibody development, as well as providing tailored solutions for targeted therapies. AI-driven protein design is revolutionizing environmental and industrial applications by facilitating green bio-manufacturing, offering a sustainable approach to reduce carbon emissions and pollution. This technology not only speeds up scientific discovery but also profoundly impacts human health, industrial production, and environmental conservation.
AI-Driven Directed Evolution of Proteins
Traditional methods of protein directed evolution rely heavily on introducing numerous random mutations, followed by multiple rounds of screening to gradually optimize protein function. This process is often time-consuming, laborious, and characterized by low success rates. However, AI-driven protein design has significantly enhanced the efficiency and accuracy of this process. By leveraging deep learning and big data analysis, AI can swiftly analyze and predict the relationship between protein structure and function - enabling the design of protein structures tailored to specific needs.
The AlphaFold project by DeepMind has made groundbreaking strides in protein structure prediction using deep learning, providing more precise structural information for protein design. Generate Biomedicines combines generative AI technology with high-throughput screening methods to swiftly design and optimize proteins with specific functions, finding wide applications in drug development and enzyme engineering. MIT's Bioengineering Lab has also developed an AI-driven directed protein evolution platform for designing highly efficient enzymes suited to specific industrial environments, contributing to more environmentally friendly and efficient bio-manufacturing processes.
AI Proteins Accelerate Chemical Drug Development
Drug development has undergone a long evolution - from the use of natural herbs in early medicine to the development of modern small-molecule drugs, and now to biologics and gene therapies. In the 20th century, scientists began using chemical methods to develop small-molecule drugs to precisely intervene biochemical pathways in disease. However, this process still requires extensive experimental screening, with an approved drug costing an average of $2 to $3 billion and taking over 10 years to develop. High costs and lengthy timelines are driven by factors like expensive, time-consuming experiments, low-quality initial hit compounds, and high attrition rates in preclinical stages. While macromolecular drugs can more effectively target specific disease mechanisms, their development and production remain costly.
AI-driven protein design is transforming drug development by accelerating timelines and significantly enhancing drug specificity and safety. With advancements in computational technology and the expansion of biological databases, AI can not only predict protein structures in 3D but also perform large-scale screening and simulations that dramatically reduce the development cycle. In particular, for antibody and small-molecule targeted drugs, AI identifies critical binding sites by analyzing the three-dimensional structure of proteins, enabling researchers to design more precise and effective drugs.
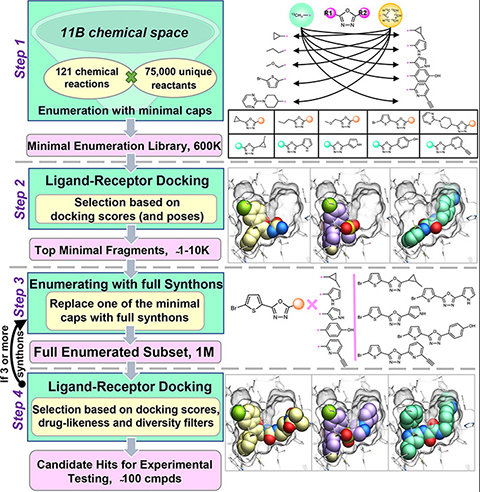
Figure 1: Syntheton-based ligand discovery in a virtual library of over 11 billion compounds
(Synthon-based ligand discovery in virtual libraries of over 11 billion compounds)
AI can analyze active sites of specific proteins in tumor cells, identifying targets associated with cell proliferation and survival. This detailed information supports the design of targeted drugs, minimizing trial-and-error processes and accelerating new drug development. Virtual screening, a highly efficient method for selecting drug candidates, enables millions of small molecules to be screened without the need for wet lab. Through computational simulations, AI evaluates how thousands of molecules combine with protein targets, selecting those with the highest therapeutic potential. For example, the VirtualFlow platform can screen over a billion compounds, identifying molecules with high affinity for KEAP1, showcasing the powerful potential of virtual screening in drug discovery.
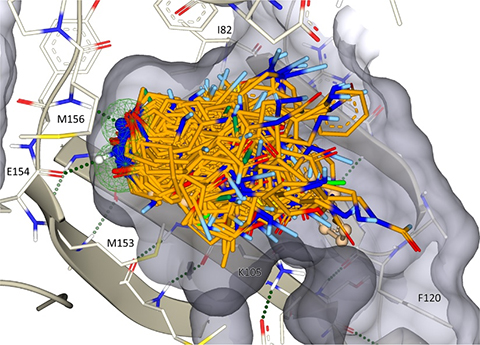
Figure 2: Fragment-based drug discovery via high-throughput docking: the ROCK1 case study
(Chemical space docking enables large-scale structure-based virtual screening to discover ROCK1 kinase inhibitors)
By integrating AI-driven protein design, generative adversarial networks, and molecular dynamics simulations, companies can rapidly screen the most effective candidate molecules - significantly reducing development timelines. AI is pushing drug development into a “Moore Law” era, where efficiency is expected to increase exponentially. With the application of deep learning and computational models, AI-designed proteins and small molecules drugs can accelerate drug development, reduce experimental trials, and optimize manufacturing, significantly shortening the time from research to market.
AI Proteins Revolutionize Antibody Development
AI excels at rapidly predicting viral protein structures, enabling the design of proteins that can elicit strong immune responses for vaccine development. This technology has already shown remarkable results in creating vaccines for influenza and COVID-19. AI-designed antibodies and proteins can specifically bind to certain disease-related molecules, allowing for precise targeting of affected areas and effectively inhibiting disease progression.
In cancer treatment, AI facilitates the creation of antibodies that tightly bind to surface proteins on cancer cells, aiding in marking or suppressing these cells without harming healthy tissues. Furthermore, AI-driven protein design can enhance the affinity and stability of antibodies, ensuring longer-lasting therapeutic effects in the body. For example, immune checkpoint inhibitors like PD-1 and CTLA-4 antibodies, used in cancer therapies, leverage AI to accurately identify and block specific protein interactions. This enhances the immune system’s ability to recognize and attack cancer cells with greater precision and efficacy.
In the field of AI-driven antibody design, companies are fiercely competing through innovative technologies and unique platforms. Companies like Absci, Generate Biomedicines, and LabGenius focus on the application of generative AI and machine learning platforms. They continuously optimize antibody affinity, stability, and specificity through high-throughput screening and feedback mechanisms, thereby rapidly screening out potential antibody drugs. The advantage of these platforms lies in their integration of massive information resources from biological databases, enabling the screening of billions of antibody variants in a short period of time. This provides precise therapeutic solutions for various indications such as cancer, autoimmune diseases, and inflammation. Atomwise's AtomNet, Schrödinger's molecular dynamics platform, and XtalPi's technology combining quantum physics with machine learning use deep learning models to accurately predict the binding sites and affinities between antibodies and targets. These technologies are widely applied in fields such as cancer and neurodegenerative diseases, aiming to improve the efficiency of antibody drug design and optimization, and offer new possibilities for targeted therapy.
Companies like Regeneron Pharmaceuticals and Amgen's Decode project leverage the fusion of genomics, big data analysis, DNA synthesis technology, and AI. Through DNA synthesis and high-throughput screening, they have automated the development of antibody drugs, accelerating the discovery of novel antibody drugs. BenevolentAI, Exscientia, and Adimab focus on rapid identification and optimization of antibody targets. Their AI platforms can analyze complex antibody structures, thereby designing antibody drugs that efficiently bind to specific targets, covering a wide range of applications including cancer and infectious diseases. Companies such as Antiverse, Iktos, and Zymeworks have also achieved significant results in the research of bispecific and multifunctional antibodies. These technologies bring unprecedented possibilities to cancer immunotherapy and precision treatment of infectious diseases. With the development of AI technology, its application in antibody design has not only expanded the boundaries of drug discovery but also significantly improved the speed and cost-effectiveness of new drug development.
AI Protein Applications in Personalized Medicine
In personalized medicine, AI-driven protein design helps identify biomarkers associated with diseases, thereby advancing the development of early diagnostic tools and personalized treatment plans. Every individual's genome and protein expression are unique, and the progression of disease may vary across different patients. By detecting changes in specific proteins within the body, AI models can analyze a patient's genetic and protein data to identify specific protein changes linked to their disease state, enabling the customization of more precise treatment strategies for each patient.
For example, by analyzing the unique protein expression profile in the tumor microenvironment of cancer patients, AI can predict how patients will respond to different cancer drugs and recommend the most suitable drug combinations. Doctors can select more accurate targeted therapies based on the specific protein expression of a patient's tumor. Through AI analysis of HER2 (human epidermal growth factor receptor 2) protein expression levels, AI can predict how a patient will respond to specific drugs (such as Herceptin), helping doctors choose the best treatment plan to improve efficacy.
Figure 3: PERCEPTION uses single-cell transcriptomics of tumors to predict patient response and resistance to therapy
(PERCEPTION: Predicting patient treatment response and resistance via single-cell transcriptomics of their tumors)
NIH researchers have developed a platform called PERCEPTION (Personalized Cancer Treatment Planning based on Single-Cell Expression) to predict cancer patients' responses to specific drugs and provide personalized, precision treatment plans. The research team uses large-scale publicly available tumor single-cell RNA sequencing (scRNA-seq) and extensive drug screening data, applying machine learning models to predict drug responses. By leveraging single-cell data for personalized treatment, PERCEPTION can identify how different subpopulations (clones) of cancer cells respond to drugs, thereby predicting a patient's drug sensitivity or resistance.
A research team from MIT's Broad Institute, Harvard University, and the Howard Hughes Medical Institute is using the CRISPR-Cas9 gene editing tool to develop more precise gene-editing methods. By optimizing the binding sites of the Cas9 protein using AI, they have enabled it to recognize a broader range of genomic regions during gene editing. This approach not only significantly reduces off-target effects but also enhances the ability of Cas9 to cut or replace gene fragments at specific locations.
AI Proteins Powering Synthetic Biology
Synthetic biology is emerging as a key engine for green manufacturing, significantly reducing the environmental impact of traditional high-carbon-emission production methods through the integration of biotechnology and AI. AI-driven protein design enables the development of more efficient enzymes and biological metabolic pathways, which can replace chemical catalysts and energy-intensive processes in traditional industries. Special enzymes designed through synthetic biology technologies can catalyze industrial reactions at lower energy costs, not only increasing product yield and purity but also reducing reliance on fossil fuels. In this process, AI helps researchers quickly screen for suitable enzyme variants and optimize their activity and stability, making the bio-manufacturing process more efficient and environmentally friendly.
The application of AI in synthetic biology is not limited to industrial manufacturing; it also plays an important role in environmental protection and food technology. AI-designed proteins can catalyze the breakdown of difficult-to-degrade waste materials, such as plastics, providing new solutions for environmental remediation. For example, specific enzymes can be used to degrade polyethylene terephthalate (PET) plastic, thereby reducing the long-term ecological impact of plastic pollution. In the food sector, AI protein design is being used to develop plant-based proteins to produce high-nutrient, low-cost foods that serve as alternatives to traditional animal proteins. This not only reduces reliance on meat production but also supports sustainability goals. By combining AI and synthetic biology, future food production and environmental management will become more eco-friendly and cost-effective, contributing to global sustainable development objectives.
Arzeda uses its AI-driven enzyme design platform to develop highly efficient enzymes for industrial processes, helping to replace traditional chemical catalysts. The company has partnered with several organizations in the fields of materials science and agriculture to advance green manufacturing and enhance the efficiency and sustainability of industrial production. Covestro employs AI to design enzyme catalysts to advance the development and production of bio-based materials, using renewable raw materials to produce polyurethane, thereby reducing carbon emissions in the chemical product manufacturing process.
Impossible Foods uses AI to design plant-based proteins, successfully developing alternative foods with the taste and texture of real meat, thereby reducing dependence on traditional meat production. This approach not only cuts carbon emissions in the agricultural sector but also reduces water and land usage. LanzaTech utilizes genetically engineered bacteria to convert industrial waste gases, such as carbon dioxide, into valuable chemicals. Its AI-driven metabolic optimization platform enhances the conversion efficiency of microorganisms, positioning the company as a leader in carbon-neutral technologies.
Genomatica focuses on using microorganisms and AI-optimized metabolic pathways to produce chemical intermediates, such as alternative ingredients for nylon and plastics. Through gene editing and AI-designed efficient enzyme synthesis pathways, Genomatica is also developing low-carbon processes for manufacturing bio-based chemicals, thus reducing dependence on petroleum.
Synbio Technologies: Bringing AI Proteins from Design to Realization
AI-driven protein design holds great promise for the future, but it also faces several challenges. For example, some protein structures generated by AI do not exist in nature, making it difficult to assess which structures are truly viable and effective. To transform AI-designed proteins into practical, usable products, a series of rigorous steps are still required, ranging from digital sequences to experimental validation.
Synbio Technologies supports the entire process from AI protein design to realization. We provide a one-stop solution that includes codon optimization, gene synthesis, expression system screening, protein purification, and functional validation to help researchers quickly translate AI-designed proteins into real-world applications. Whether it's antibody expression, large-scale production, or precise quality validation, we are dedicated to offering efficient and accurate support to drive the practical application of AI protein technology.
By partnering with us, you only need to provide the protein sequence. We handle everything from codon optimization and gene synthesis to expression system selection, protein purification, and functional validation.
-
Codon Optimization: NG Codon optimizes sequence design to improve protein expression efficiency, ensuring more rational and effective gene expression.
-
Gene Synthesis and Cloning: High-fidelity gene synthesis and cloning into any specified vector.
-
Expression System Screening: Custom expression using bacterial, yeast, insect, and mammalian hosts.
-
Recombinant Antibody Expression: Comprehensive support for all stages of antibody discovery, including antibody gene integration, de novo antibody design, antibody humanization, gene synthesis, recombinant antibody expression, monoclonal and polyclonal antibody production.
-
Large-Scale Protein Production: Flexible production scales, from micrograms to grams, to support projects of any size.
-
Quality and Functional Validation: Comprehensive testing to ensure the reliability, activity, and functionality of the final protein or antibody product.
Future Outlook
AI has already ushered in a new era in biotechnology, particularly in the field of protein research. Looking ahead, we stand at the threshold of a new age full of both opportunities and challenges. To navigate this transformation, we must engage in interdisciplinary collaboration and continually cycle through the "Design - Build - Test - Learn" process, accelerating the validation and standardization of AI-designed proteins. This will drive forward their application in areas such as drug development, personalized medicine, and synthetic biology, ultimately advancing the progress of life sciences and improving human health.
References
1. Hie, B. L. (2023). AI-driven design of symmetric protein architectures. Nature Biotechnology, 41(7), 887-894.
2. Lyu, J. (2021). Ultra-large library docking for discovering new chemotypes. Nature, 595, 597–602.
3. Jones, A. B. (2022). Fragment-based drug discovery using high-throughput docking: A case study with ROCK1. Nature Communications, 13, 6458.
4. Zhou, Y. (2024). AI-assisted protein engineering: Principles, current progress, and future perspectives. Acta Pharmaceutica Sinica B.
5. Goodman, D. (2020). Self-assembling nanocages for enhanced antibody clustering and cell signaling. Science, 370(6521), 1379-1383.
6. Arnold, F. H. (2023). From nature to industry: Harnessing enzymes for biocatalysis. Science, 382(6673), 1379-1383.
7. Liu, X. (2023). Enhanced specificity of SpCas9 variants in genome editing applications. Nature Biotechnology, 41(5), 567-574.
8. Smith, J. (2024). Predicting patient treatment response and resistance with single-cell transcriptomics. Nature Cancer, 5, 1021–1035.
9. Baker, D. (2024). Binding and sensing diverse small molecules using shape-complementary pseudocycles. Science, 385(6706), 276-282.
10. Zhou, Y. (2024). AI-assisted protein engineering: Principles, current progress, and future perspectives. Acta Pharmaceutica Sinica B.
 DNA Synthesis
DNA Synthesis Vector Selection
Vector Selection Molecular Biology
Molecular Biology Oligo Synthesis
Oligo Synthesis RNA Synthesis
RNA Synthesis Variant Libraries
Variant Libraries CRISPR Libraries
CRISPR Libraries Oligo Pools
Oligo Pools Virus Packaging
Virus Packaging Gene Editing
Gene Editing Protein Expression
Protein Expression Antibody Services
Antibody Services Peptide Services
Peptide Services DNA Data Storage
DNA Data Storage Standard Oligo
Standard Oligo Standard CRISPR Libraries
Standard CRISPR Libraries Standard CRISPR Plasmid
Standard CRISPR Plasmid ProXpress
ProXpress Protein Products
Protein Products